Health Canada has recently released The Listeria Policy (2023), a regulatory document that supersedes the previous policy from 2011. Prepared by Health Canada, in collaboration with the Canadian Food Inspection Agency (CFIA) and the Public Health Agency of Canada (PHAC), the policy focuses on the safety of Ready-To-Eat foods (RTE) offered for consumption in Canada and abroad. Imported and domestically produced food are within the scope of the Policy. The document focuses on the early detection of strains of Listeria species (Listeria spp., including biological pathogen L. monocytogenes) which may be present in the food manufacturing environment. It also addresses the verification of control measures in the context of environmental pathogen monitoring and distinguishes between food products consumed by vulnerable populations and those consumed by the general population. The policy also discusses risks associated with the manufacturing of Ready-to-Cook food. Requirements will come into effect later in October 2023.
Due to the nature of biological food safety hazards, the policy advocates for robust Good Manufacturing Practices (GMPs) and the application of risk-based methods such as Hazard Analysis Critical Control Points (HACCP) for the control of Listeria species. Examples of monitoring and verification activities include process review, surface swabbing, and food product testing. These activities are important for RTE foods that are exposed to the environment prior to packaging (with or without a prior kill-step).
PHAC reports that the incidence of Listeriosis in Canada has been stable between 2011 and 2020; 5.3 cases (per million population) have been reported in 2016; 3.3 cases (per million population) in 2017; These statistics align with Listeriosis reports from the United States and the European Union.
Definition of Ready-To-Eat Foods (RTE)
Under the policy, RTE foods are “any foods that are normally eaten in the same condition as that in which they are purchased. They are not normally further prepared before consumption, except perhaps being washed/rinsed, thawed or warmed (that is, a heat treatment achieving less than a 5-log reduction in numbers of L. monocytogenes).” Examples include fresh-cut and bagged lettuce, sliced mushrooms, grated carrots, or fruit salad. Other RTE foods linked to Listeria contamination include imitation crab meat, pasteurized milk, caramel apples, cooked diced chicken, and deli meats. A list of past foodborne listeriosis outbreaks in Canada can be accessed here.
Scope, Exemptions, and Food Safety Criteria for Ready-to-Cook Foods
The updated policy is intended for food manufacturers of human food only, not animal or pet food. The requirements do not apply to retail businesses, warehouses, or restaurants. Food manufacturers producing food for consumption in Canada or for export must comply with the Safe Food for Canadians Act and Regulations and the Food and Drugs Act and Regulations.
Under the list of RTE food exemptions, the Government of Canada lists:
- foods that are treated in a package (HPP-treated food and food processed in a retort pouch),
- hot-filled packed foods,
- low moisture foods,
- alcoholic beverages and beverages that are shelf stable.
While these foods are not subject to the Listeria Policy, other food safety requirements such as GMP procedures and a valid risk assessment as part of a PCP or HACCP plan must be developed and implemented.
Ready-To-Cook foods are also exempt from the policy provided they are properly labeled with validated cooking instructions. An adequate heat treatment parameter for Ready-to-Cook foods would achieve a 5-log reduction minimum for L. monocytogenes. Consumers of the food would, for instance, ensure that the food is cooked to an internal food temperature of 74°C/165°F. Such criteria would be recorded in the manufacturer’s food safety plan.
Furthermore, food manufacturers of Ready-To-Cook foods produced for vulnerable populations should exercise caution and increase the frequency of verification activities because their foods present a higher risk of foodborne illness over food consumed by the general population. Environmental sampling and end-product testing are additional control measures that are recommended to supplement GMP and a food safety plan for Ready- to-Cook foods that are sold refrigerated or frozen. Sampling guidelines for Environmental Monitoring are given in Figure 2.
Raw seafood and raw meat such as ceviche and tartare-type dishes are RTE and therefore subject to the rule.
Although exemptions from the Listeria Policy are published for certain foods, food businesses are required to monitor their process and report the presence of pathogenic hazards in processed foods under the Safe Food for Canadians Regulations. They must show that their food safety system will control L. monocytogenes in RTE foods. Environmental sampling and testing (surface swabs) must be carried out to verify the effectiveness of sanitation and process controls. Should the testing identify Listeria spp., a review of the food safety plan and the adequacy of the cleaning and sanitizing program should be conducted and documented.
Manufacturers should conduct environmental sampling, as described in Figures 2 to 4 (see the section on Environmental sampling and testing) to verify the effectiveness of their sanitation program and of their process controls. Findings of Listeria spp. (indicator microorganisms) may be an indication of the presence of L. monocytogenes and would require more frequent cleaning and sanitizing of the food processing environment. A review of the food safety system would also be recommended.
Control Measures
The risk level for the RTE food and the target consumer of the food dictate specific end-product testing protocols. The prescribed microbiological criteria for RTE food (general population and vulnerable population) in Canada is presented here.
Validation that the site is free from Listeria spp. is critical to the production of safe RTE foods. In addition, RTE foods in which the growth of L. monocytogenes will not occur include the following intrinsic parameters:
- pH < 4.4, regardless of aw
- aw< 0.92, regardless of pH
- combination of factors (for example, pH < 5.0 and aw< 0.94)
- frozen foods (that is, labeled ‘Keep Frozen’ on the package)
If RTE food test results are positive, immediate follow-up actions must be taken and may include recall/quarantine, investigation, additional product testing, and review of the food safety system.
The identification of Listeria spp. in the manufacturing environment suggests that GMP protocols are not effective. These sanitation conditions could lead to the presence of L. monocytogenes (high-risk food pathogen) in the environment or in RTE food. Health Canada reports that if 2 or more swab samples from the same production line test positive for Listeria spp. within a short timeframe, manufacturers should conclude that their food safety system is inadequate to control the risk of Listeria spp. in the factory.
Food safety experts agree that robust GMP programs and Environmental Monitoring Programs (EMPs) are key to controlling the spread of Listeria spp. in the food manufacturing environment. For instance, sanitary design for plants and equipment facilitates the removal of food residue. Sanitation chemicals (detergents and sanitizers) used in the rotation have been shown to dislodge biofilms (Mazaheri, 2021).
Figures 2 to 4 represent the minimum sampling and testing recommended by Health Canada for all types of RTE foods consumed by vulnerable consumers and the general population. Only Figure 2 (Sampling and Testing Requirements for Category 1 and 2 of RTE foods are shown below).
A risk-based approach determines the sampling & testing frequencies. Criteria are based on:
- The target consumer of the food (vulnerable populations)
- The shelf life of the food (could L. monocytogenes levels occur in excess of 100 CFU/g throughout the stated shelf-life)
- food that does not contain food additives or processing aids inhibiting the growth and survival of L. monocytogenes.
- food that does not receive a kill step post-packaging before distribution.
The concept of zoning (Zone 1 through 4) should be implemented in post-processing areas to detect and eliminate the presence of Listeria spp. The Listeria Policy recommends that sampling be done 3 hours after the beginning of production. 1 to 10 samples should be collected (individual swabs or one composite of 10 samples). Both food contact and non-food contact sites must be considered. In addition, ATP or conventional micro swabs can be used post-sanitation or at pre-op to verify the adequacy of the sanitation program.
EMP procedures should be managed through CAPA (corrective actions and root cause analysis) so that RTE food manufacturers can react to presumptive and confirmed positive environmental test results with appropriate actions. Trend analysis is also recommended for continuous improvement in EMP monitoring and corrective actions. Trending may uncover the presence of Listeria spp. in areas that are distant from food manufacturing areas. It is widely accepted that the identification of Listeria spp. from non-food contact surfaces usually precedes detection from food contact surfaces. Being able to identify potential harbourage sites in zones 3 and 4 would trigger correction actions to prevent the spread of contamination to zones 1 and 2.
While swabbing and testing for Listeria spp. is an acceptable and robust approach to ensuring food safety, manufacturers who choose to test Food Contact Surfaces (Zone 1) for L. monocytogenes must take corrective actions and hold produced lots of RTE food while lab results are pending. Finished product testing for L. monocytogenes should also be performed if L. monocytogenes is detected on food contact surfaces (refer to flow charts in Figures 2 and 3).
Alternative food safety measures
In keeping with the concept of Hurdle Technology, food additives, food processing aids, and post-packaging treatments may be used to control pathogenic hazards in RTE food and lower the risk of contamination and growth. For instance, Nisin has been approved as a permitted preservative in Canada since 2018. It may be used in RTE meats and RTE smoked fish as well as sausage at a concentration of 25 ppm. Modified atmosphere packaging (carbon dioxide) can also be used in conjunction with nisin to inhibit L. Monocytogenes (USDA, 2015).
Figure 2: Sampling guidelines for food contact surfaces, Category 1 ready-to-eat foods, and Category 2 ready-to-eat foods specifically produced for consumption by vulnerable populations
(Source: https://www.canada.ca/en/health-canada/services/food-nutrition/legislation-guidelines/policies/listeria-monocytogenes-ready-eat-foods.html)
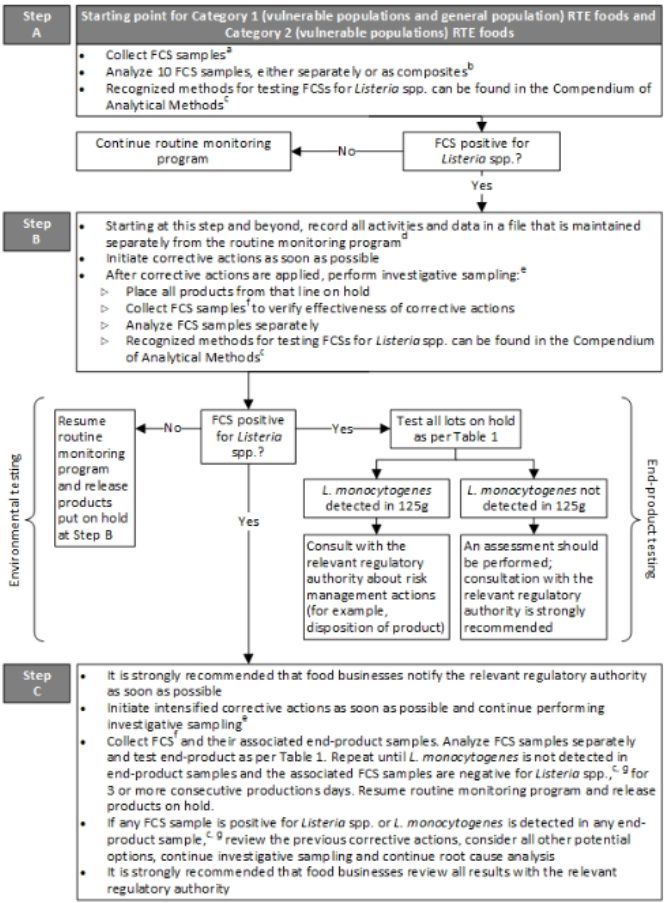
- With the zone concept, Zone 1 includes FCSs where RTE foods are exposed to the environment prior to packaging. The number of meaningful sampling sites (10 recommended) selected on each processing line should depend upon the complexity of the lines. Details on environmental sampling are described in method MFLP-41 (Health Canada, 2010).
- If analyzing FCS samples as composites, a maximum of 10 FCS samples should be combined.
- The ‘application’ section of the method should be appropriate for the intended purpose (for example, MFHPB and MFLP methods; Health Canada, 2021a).
- The records should include information on corrective actions, investigative sampling, end-product testing, and risk management actions (for example, disposition of product).
- Investigative sampling will assist in finding and eliminating the source of Listeria spp., particularly if there is a harbourage site from which Listeria spp. or a specific subtype of L. monocytogenes is repeatedly isolated.
- At a minimum, the FCS sites in the routine monitoring program should be included. The number and location of samples should be adequate to verify that the entire line is negative and under control.
- A qualitative method for L. monocytogenes (that is, a detection method using enrichment) should be used for end-product testing. Recognized methods for end-product testing can be found in the Compendium of Analytical Methods (Health Canada, 2021a).
References
Policy on Listeria monocytogenes in ready-to-eat foods (2023): Overview
Foodborne Listeriosis in Canada
Environmental Sampling and Testing Flow charts
Sirocco Consulting is a full-service consulting firm knowledgeable about food safety compliance/certification process. We are experienced in all levels of Food Safety Compliance and Certification (Canada, US), and are qualified to provide Food Safety Consulting for GMP, HACCP, SQF, FSMA, and Canadian SFCR. Contact us to request more information.