I have had the pleasure of working with Karine for over a year now. She helped our company pass our first SQF audit Ed. 8.1 with a score of 94. For our recertification audit, which was on SQF Ed. 9.0, Karine visited our site and performed a gap assessment. She was able to identify a lot of areas for improvement and with her help our company passed the audit and achieved a score of 96. Karine is really knowledgeable on SQF. She is easy to work with and extremely helpful.
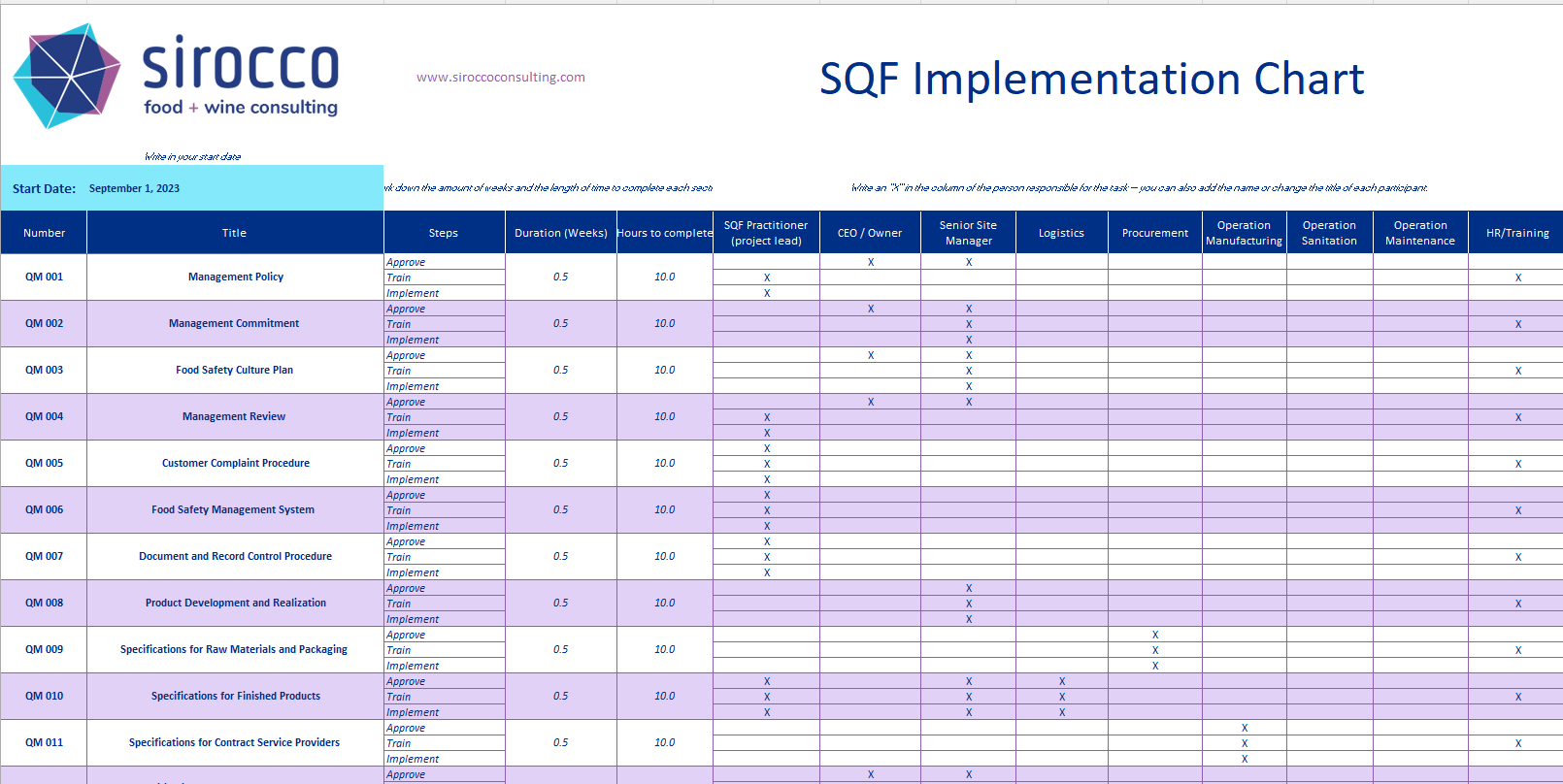
Charting the Course to SQF Success: 7 Essential Steps to Certification
The Sirocco Difference

Able to assist small and medium food companies in Canada and the United States in multiple languages – English, French, Spanish, Mandarin, Cantonese.

Certification Fast-Track! Our clients save weeks, even months, when becoming certified.

Training provided & document templates written by a certified SQF trainer and consultant with 25 years of experience. The Sirocco team holds the highest credentials (Master of Food Science, SQF designations)

Affordable SQF and HACCP implementation. Save money with affordable courses and compliance documents (HACCP, SQF, Canadian PCP plans).

No subscription is needed*. Instant Download. Easy DIY, no special tech skills needed. Quick and easy access to a food safety expert (30 min, 1 hour Zoom consultations)

Need personal support? Utilize our 1-to-1 consulting. Personal support with experts is always accessible.
Questions? Ready to get started? Drop us a line!

SQF Gap Assessments
All SQF site certifications start with a gap assessment to determine how fast your business may implement the SQF Code. The goal of the assessment is to:
- Gauge the food safety culture of the site, and meet with the SQF team.
- Evaluate the facility readiness to ensure GMP compliance.
- Identify what food safety programs the site currently maintains.
- Confirm the regulatory scope of the facility and corresponding documentation
- Assess the “gaps” or the missing elements that are required for SQF certification.
- Review and discuss training needs
Interested in a free Zoom consultation with our consultant Karine Lawrence? Email us.
How Sirocco Can Help after the Gap Assessment
- Sirocco can provide a quotation to develop your entire SQF manual or Procedures and assist with training and full implementation.
- Alternatively, you can purchase an SQF template bundle and implement SQF directly yourself with no or little involvement from our team.
- SQF certification can be achieved very fast if your facility is already HACCP certified. If you need a food safety plan, a manual of procedures and training, it may take 6 months or longer to achieve certification.
- Sirocco can also develop food fraud vulnerability assessments, and food safety plans (HACCP, FSVP and Preventive Controls for Human Food, Canadian PCP Plans) and can perform your internal audits.
- We can also train your team on becoming SQF practitioners and Internal Auditors. Check our store for details.
- Our clients from Texas achieved SQF certification within 6 months. Read our case study here.

Next Steps
Are you committed to achieving SQF within 6 months?
Do you need help or prefer the DIY approach?
Do you need the help of our SQF and regulatory experts?
Interested in a do-it-yourself option?
What Clients Are Saying
We successfully passed our initial HACCP certification audit, without a single finding! While this seemed quite surprising, I then realized that this was due to the thorough gap assessment provided by Sean Xia at Sirocco Consulting, along with additional documentation support.
Sean inspected our facility and dug through all of our systems and records in such detail as to be able to expose all of our issues. We were then provided with our initial HACCP plan and a set of GMP documentation that we were able to edit and integrate with our existing documentation. But these documents were not just provided to us, they were each individually reviewed in a handover that provided further insights. And Sean also made himself available for future questions – such amazing support!
As we proceeded to work through implementation of the new GMP’s and to address all items in the gap assessment, I felt quite confident – albeit a bit trepidatious – about the pending audit. However, on the audit date, things went so much more smoothly than I ever could have imagined.
Thank you, Sirocco!
My company needed to create a more robust Preventative Control Plan than what we had, and I am so thankful to have found Sirocco Food + Wine Consulting. Their templates were just what I needed, and Karine and Sean were readily available to answer my questions and provide guidance when necessary. Karine and her team are highly competent, knowledgeable, friendly, and prompt to respond with help. I can’t recommend them enough!
Karine is such a wonderful teacher. As a student for the PCQI hybrid course I learned a lot from the virtual course but was left with a lot of questions. I was looking forward to the instructor-led course to get some more practice and answers to my questions. Karine was clear and thorough in her explanations. Her consulting business experience gave her a plethora of examples to draw from. I was impressed by her ability to address my questions and give me resources to continue my study. She is very well versed in the regulations required and is up to date on any changes or new publications that may influence your business. She was very accommodating to my needs. I highly recommend taking a class from Karine. She is an expert in her field. I left the class feeling confident in my ability to act as a PCQI and make a positive impact on my company.
I have been very pleased and satisfied with the SQF Bundle edition 9 developed by Karine Lawrence from Sirocco Food + Wine Consulting.
All procedures (templates) are professionally and beautifully written, saved us a tremendous amount of time, and enabled us to achieve our goal of gaining an excellent score in the audit.
Karine is very knowledgeable, and it has been a pleasure working with her. I highly recommend the SQF Bundle and her consulting services.
“Karine taught the PCQI course clearly, and at a good pace that didn’t feel too fast. She asked insightful questions that sparked discussions and helped broaden the expertise of those involved in the class, I would recommend it to others.”
We have been using the expertise of Karine in the implementation of the SQF program and have always been completely satisfied with her help and guidance. She did an excellent job by directing us to the right resources and to obtain an excellent score in the SQF audit. We have also used the SQF templates available with Sirocco Food Safety Consulting to write our SOPs to comply with the SQF codes. The Spanish templates were very useful in creating SOPs in Spanish to cater most of our employees. I’m happy to recommend the services of Sirocco Food Safety Consulting for your SQF certification needs.
“As a Faculty Instructor in the area of Food Technology, a key course taught in the BCIT programme, is sensory evaluation. Most of it is a hands-on approach with physically applying and calculating the correct statistics associated with the testing. When Karine reached out to me with the opportunity to try out a sensory software programme from Tastelweb©, it enabled the students to look at tests such as QDA and Pivot Profiling Tests in more detail. Its ease of use, ability to conduct on a smartphone and automatic calculation of statistical analysis was a winner with the students! This programme also created very good impactful visuals for easy interpretation even for the non-scientist. Karine was also available to provide backup support for set up and interpretation of the results. This programme provides the flexibility of usage without a contract, especially to a smaller business where cost is a big factor. In addition, exposure to software programmes like Tastelweb© helps build a good portfolio for students graduating in a competitive industry.”
Karine is known in the food industry as an expert in Food Safety: conducting food safety related trainings, assisting companies develop and implement their food safety programs, and a wine connoisseur. I had the privilege to attend several of her trainings, which are practical and easy to understand. Our company needed assistance on SQF gap assessment and she provided valuable advice and information for us to ace our SQF Certification. I recommend her to companies which are building their programs and are committed to start right and want to maintain an excellent Food Safety System.
When we decided to implement the SQF program in our facility we knew we would have to develop a lot of new procedures and forms. We reached out to Karine Lawrence of Sirocco Consulting and with her guidance and templates, we were able to smoothly implement the code. The template package was very complete and easy to follow; each document made reference to the element of the code being covered making it easy to track our progress and if we had any questions Karine was quick to answer and provide examples. We had our desk audit recently and the auditor was happy to see how complete our documentation was, the template package and Karine’s support were key in this accomplishment.