The US Congress passed FSMA (Food Safety Modernization Act) on December 20th, 2010 and President Obama signed it into law on January 4th, 2011.
Implementing Preventive Controls for Food Safety
Section 103 of the act is about implementing Preventive Controls for food safety. The “rule” —cGMP (Current Good Manufacturing Practices) and Hazard Analysis and Risk-Based Preventive Controls for Human Food rule (“PCHF” rule, 21 CFR Parts 1, 16, 106, 110, 114, 114. 117, 120, 123,129, 179 and 211) — requires registered facilities, unless exempt, to:
- Have a written food safety plan
- Perform a hazard analysis
- Implement risk-based preventive control measures
- Conduct Monitoring
- Perform Corrective Actions
- Verify the effectiveness of these preventive controls
- Maintain records
Compliance starts this coming September for small businesses (fewer than 500 full-time equivalent employees).
Preventive Controls Qualified Individual
While sharing some commonalities with HACCP, Preventive Controls go beyond Process CCPs. The Preventive Control Qualified Individual (PCQI) has the mandate to develop or oversee the preparation of the food safety plan, validates the preventive controls, reviews records and is responsible for re-assessing the food safety plan a minimum of every 3 years unless changes are required. One way to become a PCQI is to attend an FSPCA course taught by an FSPCA lead instructor. Another less formal way to qualify as a PCQI is through job experience and knowing the requirements (i.e; having read and understood the 600+ page rule!).
Contents of a food safety plan include:
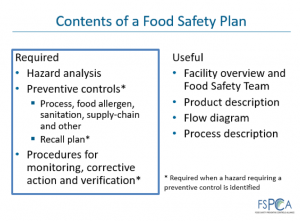
The rationale given by the US FDA for the Preventive Controls requirements is that food companies with HACCP plans have not put the due diligence into implementing and monitoring food safety risks. As a result, some critical points such as supply chain, allergen and sanitation, that would have required monitoring and verifying under HACCP, have been relegated to a Control Point (CP) under pre-requisite programs. This behaviour has led to food recalls.1
Are you looking for information on HACCP? Paperless HACCP Plans and Food Safety Software are discussed here.
FDA is specific about the food safety plan and implementation records that the business must maintain.2 Maintaining these records will be significant.

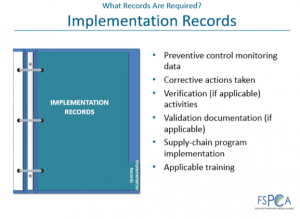
“All monitoring and corrective action records must be reviewed within seven (7) working days from the time they were created. This should preferably be done prior to releasing the product. Verification records, including calibration, product testing, environmental monitoring and supplier program records must be reviewed in a reasonable time and performed or overseen by a PCQI. When issues are identified during the review, corrective action is required.
The food safety plan must also be validated using scientific principles and data, use of expert opinion, [through] in-plant observations or tests, [or/and by] challenging the process at the limits of its operating controls. Plan validation is restricted to Process Preventive Controls but may also include the validation of other PCs such as Sanitation Preventive Controls and must be performed or overseen by the Company PCQI.”
Become a PCQI with Instructor-led Workshops
From a practical and financial perspective, it will be difficult for outside consultants to act as food safety plan verifiers. It is therefore preferable that companies invest in training their own staff to become PCQIs and back-up PCQIs. The instructor-led workshop provides participants with a complete and up-to-date course manual including numerous and documented hands-on case studies that provide a nuanced understanding of how the new requirements are interpreted and what possible record-keeping options may look like. Although food safety records are not required to follow a specific format under the regulations, it makes sense to follow the document format from the only FDA-recognized PCHF curriculum, all the more since FDA employees receive training on the exact same training materials. Receiving PCQI training is therefore a good investment and speaks to the business level of food safety culture. Being proactive in learning how to implement the PCHF requirements will better your odds at passing your first FDA inspection.
References
- Food Safety Modernization Act 2010 Update September 2013 Webinar, SAI Global
- FSPCA Preventive Controls for Human Food Participant Manual (2016 Edition)
About FSPCA Lead Instructor, Karine Lawrence
Karine Lawrence, Sirocco Principal, has worked for the Canadian and US wine and food industry for over sixteen years and brings an extensive working knowledge in Food Safety and Quality Management Systems (GMP, HACCP, SQF, Preventive Controls for Human Food) including regulatory compliance, program implementation and auditing. Karine holds a Master of Food Science degree as well as a diplôme d’ingénieur (specialized in agri-food industries). She is a FSPCA Lead instructor, an approved Food Safety Advisor (FSA), Certified Safe Quality Food (SQF) trainer/consultant and Certified HACCP professional (CHA-ASQ, CCHP).





