The US FDA announced the New Era for Food Safety blueprint in July 2020 in an effort to “enhance traceability, improve predictive analytics, respond more rapidly to outbreaks, address new business models, reduce contamination of food, and foster the development of stronger food safety cultures.”
One of the keystones of this food safety initiative is the implementation of the final rule on Requirements for Additional Traceability Records for Certain Foods (known as the “Food Traceability Final Rule”1 which can be found in section 204(d) of the FDA Food Safety Modernization Act (FSMA)). Under FSMA, companies that manufacture, process, pack, or hold foods included on the Food Traceability List (FTL) must maintain enhanced traceability records. The new requirements are designed to allow for faster identification and rapid removal of potentially contaminated food from the market, resulting in fewer foodborne illnesses and/or deaths. Compliance date is January 20, 2026.
Frank Yiannas, Deputy Commissioner for Food Policy and Response at the U.S. Food and Drug Administration, believes the rule is “a landmark action that […] will change the way government and industry work together to keep food safe and, in so doing, will help save lives for generations to come.”
FSMA (US FDA) Traceability Requirements
The “standardized data-driven approach” to traceability requires food businesses to maintain key data elements (KDEs) – such as lot numbers and harvest locations – for critical tracking events (CTEs) along the food supply chain and to send required KDEs to the next stakeholder who is receiving the food.
The new rule complements existing regulations for Food Recall outlined in the Preventive Control for Human Food (PCHF) rule. The PCHF rule prescribes that if a significant food safety hazard is identified in a food safety plan risk analysis, a facility must have a written recall plan that describes the procedures to perform a recall of the product. The recall plan must include procedures to notify consignees, including public notification when required, to conduct inventory reconciliation checks, and to appropriately dispose of recalled product so that it doesn’t inadvertently re-enter commerce. A traceability program is therefore required to support the recall plan.
What food products are on the Food Traceability List?
The new Traceability Rule is intended to track the movement of high-risk foods more efficiently. Food products that are prone to contamination with pathogenic Salmonella and Listeria monocytogenes include produce, cheeses, eggs, nut butter, seafood, and Ready to Eat (RTE) deli salads. These foods have been identified by FDA as causing the most outbreaks of food-borne diseases and recalls (See FSMA dashboards). As the Institute of Food Technologists puts it, the use of Key Data Elements by industry and government allows a simple, fast, and effective means for “route-through-supply-chain” discovery prior to digging deeper into more detailed business data. Such initiatives are enabled by data-query systems.
What are Critical Tracking Events (CTEs)?
As per the regulations, The Critical Tracking Events in the final rule are harvesting; cooling (before initial packing); initial packing of a raw agricultural commodity other than a food obtained from a fishing vessel; first land-based receiving of food obtained from a fishing vessel; shipping; receiving; and transformation of the food.
All records required under this rule, along with any information required to understand the records, must be made available to the FDA within 24 hours after a request is made (or within a reasonable time to which the FDA has agreed).
The law makes exemptions for certain business entities (small farms, stores and, food service), as well as some foods that are treated to reduce contamination and produce that is rarely consumed raw. Use the FDA exemption flow chart to determine which requirements apply to your business.
Resource Guide to find out what traceability data to keep.
Who is subject to the Traceability Rule?
Canadian SFCR Traceability Requirements
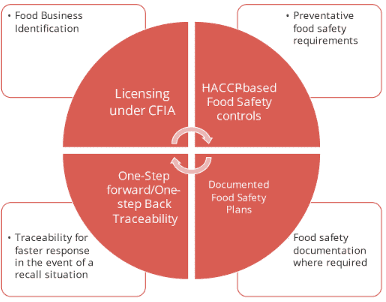
Federal traceability regulations for food in Canada are based on the international standard established by Codex Alimentarius – tracking of food forward to the immediate customer and back to the immediate supplier. Requirements are found in Part 5 of the Safe Food for Canadians Regulations 3. Traceability has been identified as one of the 4 key elements of the Safe Food for Canadians Act and Regulations.
4 Key Food Safety Elements
Food that is traded across inter-provincial borders must be identified as follows:
- common name
- lot code or another unique identifier; and
- name and principal place of business of the entity by whom or for whom the food was manufactured, prepared, produced, stored, packaged or, labeled.
Food businesses are expected to trace the food one step back to the immediate supplier by indicating the date food was received and the name and address of the supplier who provided it. In addition, companies must trace food one step forward by indicating the date products were sold to a customer and name and address of the entity the food was shipped to. Traceability requirements also include foods sold at retail.
Traceability documents must be made available upon request to the Canadian Food Inspection Agency (CFIA) in English or French and within 24 hours of the CFIA making the request or within a shorter period of time if the CFIA believes there is a risk of injury to human health. Use the CFIA traceability interactive tool to find out which requirements apply to your business.
References
- FSMA Final Rule on Requirements for Additional Traceability Records for Certain Foods
- Food Safety News – FDA’s traceability rule is a game changer for food safety
- Safe Food for Canadian Regulations – Traceability for food
Sirocco Food + Wine Consulting uses Tastelweb® software for the design of consumer and sensory questionnaires, data entry, processing of results, and reporting. Five tests are currently offered: Consumer Tests, QDA®, Mapping, Triangle, and Pivot tests. Individual tests can be purchased through the store (no contract needed). Sirocco Consulting and Tastelweb® will be at the Pangborn Sensory Symposium this summer.





