What is CAPA?
In order to understand the usefulness of CAPA, we must first introduce the PDCA cycle. The Plan-Do-Check-Act (PDCA) cycle is a four-step quality management process that was introduced by Dr. W. Edwards Deming in 1950. A graphical representation of the Deming wheel is represented below. This model is commonly used by project teams and is at the core of all food safety/quality management systems and continuous improvement initiatives.
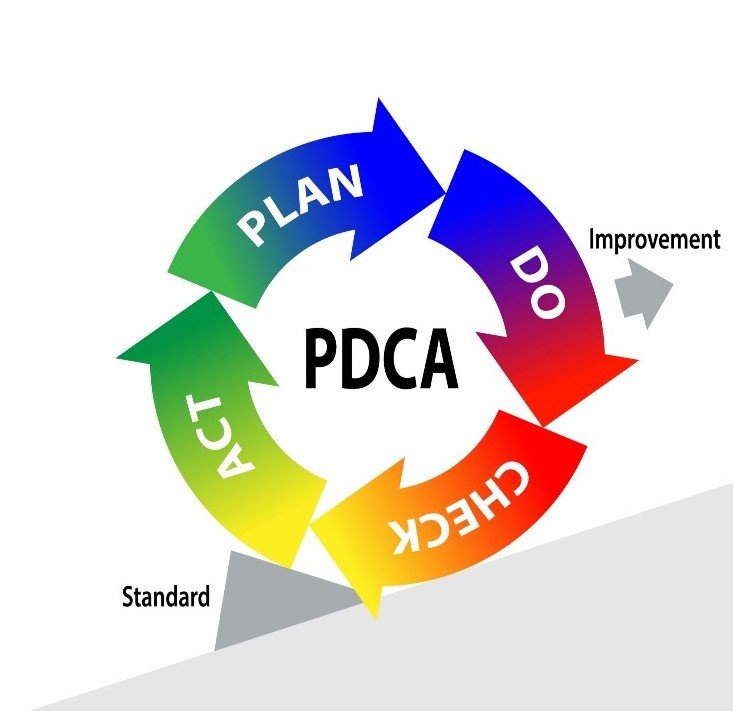
Corrective Action/Preventive Action, CAPA for short, is a tool that enables the systematic investigation of a non-conformance in order to identify its true root cause(s). In a second phase, a corrective (preventative) action plan is developed to eliminate the source of the issue and prevent its recurrence. CAPA is a tool that can be utilized during the “Check” and “Act” stages of the PDCA cycle. Without a robust CAPA problem, there would be no process improvement.
SQF Code Manufacturing Requirements – Edition 8.1
2.5.3 Corrective and Preventative Action (Mandatory)
2.5.3.1 The responsibility and methods outlining how corrections and corrective actions are determined, implemented and verified, including the identification of the root cause and resolution of non-compliance of critical food safety limits and deviations from food safety requirements, shall be documented and implemented.
2.5.3.2 Records of all investigation and resolution of non-conformities including their corrections and corrective action shall be maintained.
What are Corrections?
A correction is an immediate action taken to eliminate a detected non-conformity.
In the context of FSMA2 , a correction is an action to identify and correct a problem that occurred during the production of food, without other actions associated with a corrective action procedure (21 CFR 117.3).
Corrections refer to actions related to minor and isolated problems that do not directly impact food safety. Examples: closing a site entrance door to prevent pest entry; re-cleaning a food contact surface following a pre-op inspection or a facility audit; reprocessing a food product to reach a critical limit and ensure food safety.
What are Corrective (Preventative) Actions?
Corrective (Preventative) Actions describe an action to eliminate the cause of a detected non-conformity or other unwanted situation and includes root cause analysis. Actions are “preventative” when they aim to address repeated observations (“patterns” or “trends”) that may lead to product or process deviations.
The US FDA Preventive Controls for Human Food rule defines Corrective Actions as Procedures that must be taken if preventive controls are not properly implemented (21 CFR 117.150(a)(1)).
In the context of HACCP, the FAO3 describes Corrective Actions as “Any action to be taken when the results of monitoring at the Critical Control Point (CCP) indicate a loss of control” (ex: placing product on hold).
CAPA is a mandatory element of SQF. The SQF code Manufacturing requires that a designated employee or team take immediate correction and corrective/preventive action when food products or manufacturing processes deviate from specifications and requirements. This process must be documented. It is recommended to follow a Standard Operating Procedure (SOP) format to document the site’s CAPA process. In your procedure, identify “responsibility” and “methods”, and reference your food safety plans and CCP procedures. When opening corrective action reports (“CARs”), identify the root cause(s) and the method your team has used to determine it/them. Non-conformities must be addressed systematically within an appropriate completion timeframe.
When to open a CAR?
The SQF practitioner or designate will raise a Corrective Action when:
- CCP failures requiring corrective actions
- Non-conformities are reported following an internal or external food safety audit or regulatory inspection.
- Process monitoring identifies trends that must be addressed.
- Customer complaints are received and identify significant food safety/quality issues.
- The site experiences a recall or a market withdrawal or receives a warning letter from a regulatory agency.
- Mock recall exercises identify traceability gaps in the process that must be corrected.
Root Cause Analysis (RCA)
There are a number of techniques that may be used to perform RCA4 . Simple methods that are often suggested during food safety training are the 5 Why’s method and the Fishbone diagram. When conducting RCA, avoid these common pitfalls:
- Avoid “Death by CAPA” – only perform RCA on serious deviations and follow the SQF code requirements. The site may need to revisit its CAPA program if your CAR log shows hundreds of active CARs at any given point.
- Relying solely on in-house expertise. The problem may require outside help.
- Confusing cause and effect. Identify the real source (“cause”) of the problem, not the symptom (“its effect”). If using the Fishbone diagram, brainstorm all possible factors for a complete analysis.
- Assuming that a problem is the result of a unique root cause where it may be more complex. If using the 5 Why method, the team may investigate multiple lines of questioning to explore the root causes of a non-conformity.
- Separate the people from the problem. Identify which food safety system element the team needs to review (ex: is your employee training procedure effective?).
Validation of Root Cause and Closing CARs
A Corrective Action Report is closed when a review of completed corrective actions identifies that a plan has been fully implemented and is effective. This process may involve trending and conducting inspections and audits. The review must conclude that process indicators and product characteristics meet specifications and are back under control. It must also confirm that the implementation of corrective actions did not create further issues that could affect the safety of the manufacturing process.
For a more in-depth look at RCA, consult and download PEW’s Guide for Conducting a Food Safety Root Cause Analysis.5

